Colloidal particles have a complex structure. They consist of nuclei and adsorbed and attracted ions. Let us consider the structure of a colloidal silicic acid particle, which was formed as a result of the interaction of very dilute solutions of sodium silicate and hydrochloric acid (Na2 Si03 + 2HCl = H2 Si03 + 2NaCl). If Na2 Si03 is in excess, then silicic acid does not precipitate, but a transparent colloidal solution of H2 Si03 is formed. The core of a colloidal particle is neutral; it consists of m H2Si03 molecules. n Si03 2- ions are adsorbed on the surface of the core; these are potential-determining ions, since they determine the charge of the particle of the colloidal solution.
Adsorbed potential-determining ions attract ions of the opposite sign from the solution - counterions. In this case, these are Na+ ions, and part of them 2(n - x) is adsorbed on the particle. Adsorbed Si03 2- ions together with Na+ counterions form an adsorption layer. The other part of the 2xNa+ counterions is in the liquid phase and forms a mobile diffuse layer.
The core together with the adsorption layer is called a granule.
In our example, the granule is negatively charged, since the adsorption of Si03 2- ions occurs more strongly than Na- ions.
The colloidal particle, together with the counterions of the diffusion layer, is called a micelle. This is a separate particle of a colloidal solution.
Micelle (colloidal particle)
Coagulation(lat. coagulation- coagulation, thickening, enlargement) - the combination of small dispersed particles into larger aggregates.
Coagulation is a complex of chemical and physical interactions between negatively charged colloidal particles and cations, i.e. positively charged chemical reagents. It uses various forces of repulsion and attraction, which provide stability or, conversely, instability of the colloidal suspension, namely:
- electrostatic repulsion forces
- Brownian motion
- Van der Waals gravity forces
- force of universal gravity
Coagulation destabilizes the colloidal suspension through two different mechanisms:
1. Neutralization of charge
Positively charged coagulants neutralize the negative charge surrounding colloidal particles. When the charge around each particle is neutralized, they gradually move closer together, decreasing their effective radius, and eventually become unstable and can collide with each other. When they collide, particles are connected to each other due to hydrogen bonds or, for example, van der Waals forces, forming large masses, or flakes. The agitation energy applied in the cleaning process increases the number and frequency of these particle collisions, increasing solid agglomeration and promoting floc formation.
2. Chemical bonding
The formation of flocs is facilitated by the polymeric nature of coagulants. Their long molecular chains pick up agglomerated particles and form bridges from one surface to another, binding together individual flakes into large, easily removable masses.
Schulze-Hardy rule.
The coagulating ability of a coagulating ion increases with increasing its charge (Schulze's rule).
The ability of dispersed systems to maintain a certain degree of dispersion is calledaggregative stability.
Particles dispersed phases resist sticking together due to different mechanisms. This ability is due, firstly, to the formation of a double electrical layer on the surface of the particles of the dispersed phase, which ensures electrical stabilization of the dispersed system. Secondly, the molecular adsorption stabilization mechanism operates, which consists in the formation around the particles of adsorption layers consisting of molecules of the dispersed medium and substances dissolved in it . Thirdly, there is a kinetic factor of stability - a low frequency of collisions of dispersed particles.
Sols (colloidal solutions) differ from coarsely dispersed and molecular systems in their aggregative instability, so they change both over time and with the addition of various substances.
The essence of the mechanism for purifying water from suspended colloidal particles is to disrupt the equilibrium state of the system - eliminating the balance of forces that do not allow the particles to settle.
To achieve this goal, it uses the process of coagulation of colloidal impurities (simply - water coagulation).
Coagulation - the process of colloids sticking together into larger aggregates, which occurs as a result of their collisions during Brownian motion, mixing or directed movement in an external force field, or the addition of coagulants. In this case, a sediment forms - coagulate.
Coagulants (usually soluble iron or aluminum salts) intensify the coagulation process. The introduction of these substances into water promotes the formation of a new, slightly soluble phase (as a result of hydrolysis - the interaction of a substance with water). Thus, the coagulation process consists in the progressive enlargement of particles and a decrease in their number in the volume of the dispersion medium.
Coagulation can be slow or fast. With slow coagulation, only a small part of the collisions of colloid particles causes them to stick together, and the coagulum does not fall out. With rapid coagulation, each collision is effective and causes particles to stick together, and a precipitate gradually forms in the colloidal solution.
The minimum concentration of the dosed substance (electrolyte or non-electrolyte) that initiates the coagulation process in a system with a liquid dispersion medium is called the coagulation threshold. Under certain conditions, coagulation is reversible. The process of turning the coagulum back into sol is called peptization, and the substances that provoke this process are called peptizers. Peptizers, being stabilizers of dispersed systems, are adsorbed on the surface of particles, weakening the interaction between them, resulting in the disintegration of aggregates. The return to the primary state is especially effective when surfactants are introduced into the medium, which reduce the surface interfacial energy and facilitate dispersion.
Coagulation using iron salts
Let us consider what processes occur when iron (III) sulfate is added to a colloidal solution. This coagulant in aqueous solution dissociates into iron ions and sulfate ion:
Fe 2 (SO 4) 3 → 2 Fe 3+ + 3 SO 4 2-
Fe 3+ + H 2 O ↔ Fe(OH) 2+ + H +
Fe(OH) 2+ + H 2 O ↔ Fe(OH) 2 + + H +
Fe(OH) 2 + + H 2 O ↔ Fe(OH) 3 ↓ + H +
Fe 3+ + 3H 2 O ↔ Fe(OH) 3 ↓ + 3H +
Micelle - structural unit lyophobic (weakly interacting with liquid) colloids that do not have a specific composition. Schematically, its structure using the example of an iron (III) hydroxide micelle can be depicted by the diagram:
(mFe(OH) 3 2nFe(OH) 2+ (2n - x) SO 4 2- )2x+ xSO 4 2-
 A microcrystal of iron hydroxide, forming a colloidal particle (see figure), selectively adsorbs from the environment ions identical to the ions of its crystal lattice. Depending on the chemical composition of the solution (excess sulfate ions or excess iron ions), the microcrystal acquires a negative or positive charge. Such a charged crystal is called the micelle core, and potential-determining ions impart this charge to it.
A microcrystal of iron hydroxide, forming a colloidal particle (see figure), selectively adsorbs from the environment ions identical to the ions of its crystal lattice. Depending on the chemical composition of the solution (excess sulfate ions or excess iron ions), the microcrystal acquires a negative or positive charge. Such a charged crystal is called the micelle core, and potential-determining ions impart this charge to it.
The electric field of the charged surface of the crystal attracts from the solution counterions - ions that carry an opposite charge. An electric double layer is formed at the phase interface, the thickness of which is determined by the outer boundary of the counterion cloud.
The electric double layer consists of adsorption and diffuse parts. The adsorption layer includes potential-forming ions and part of the counterions adsorbed on the surface of the core. The diffuse layer is completed by the remaining counterions in an amount that contributes to the electrical neutrality of the micelle.
The double electrical layer surrounding the colloids, under the influence of coagulants (electrolytes), is reconstructed: counterions begin to be forced out from the diffuse to the adsorption part, and the thickness of the entire electrical layer decreases over time to the thickness of the adsorption layer. Dispersed particles fall into an area of mutual attraction, and rapid coagulation occurs.
Coagulation using aluminum salts
 Most often, 18-aqueous aluminum sulfate crystal hydrate - Al 2 (SO 4) 3 - is used for water purification by coagulation at domestic water treatment stations and swimming pools. 18H2O.
Most often, 18-aqueous aluminum sulfate crystal hydrate - Al 2 (SO 4) 3 - is used for water purification by coagulation at domestic water treatment stations and swimming pools. 18H2O.
The processes that occur when aluminum salts are added to water are similar to those described above when iron salts are added:
Al 3+ + H 2 O ↔ Al(OH) 2+ + H +
Al(OH) 2+ + H 2 O ↔ Al(OH) 2 + + H +
Al(OH) 2 + + H 2 O ↔ Al(OH) 3 ↓ + H +
The overall hydrolysis equation is:
Al 3+ + 3H 2 O ↔ Al(OH) 3 ↓ + 3H +
The formation of aluminum hydroxide precipitate occurs at pH values in the range from 5 to 7.5. At pH< 5 осадок не образуется. При рН >8.5, the resulting aluminum hydroxide dissolves to form aluminates.
Al 2 (SO 4) 3 + 6 NaOH = 2 Al (OH) 3↓ + 3 Na 2 SO 4
Al(OH) 3 + NaOH = Na or (NaAlO 2 . 2H 2 O)
Modern coagulants
Coagulants based on aluminum polyoxychloride are becoming increasingly common in water treatment and wastewater treatment processes.
The advantages of these coagulants compared to aluminum sulfate:
Delivery in the form of solutions, which makes their use more convenient (no need to dissolve);
Higher percentage of active substance;
Obtaining purified water of higher quality;
Reducing the volume of secondary waste;
Low residual aluminum content (< 0,2 мг/л);
No pH adjustment required;
Wide operating temperature range.
Technical characteristics of such coagulants produced by JSC AURAT:

Contact coagulation
 One of the cleaning options using the coagulation method is contact coagulation. Contact coagulation occurs on the loading grains of vertical pressure filters of mechanical cleaning. In this case, the coagulant is introduced directly in front of the mechanical filter. The loading grains and particles adsorbed on them serve as coagulation centers. The process of flocculation in this case is significantly accelerated.
One of the cleaning options using the coagulation method is contact coagulation. Contact coagulation occurs on the loading grains of vertical pressure filters of mechanical cleaning. In this case, the coagulant is introduced directly in front of the mechanical filter. The loading grains and particles adsorbed on them serve as coagulation centers. The process of flocculation in this case is significantly accelerated.
The coagulation process occurs at a higher speed and the absence of the need for settling tanks for the formation and sedimentation of sludge flocs are the undoubted advantages of contact coagulation.
The disadvantages of contact coagulation include accelerated contamination of pressure filters and the need for frequent regeneration of the load, as well as the danger of reagent leakage in case of incorrect selection of the coagulation/filtration mode.
To check whether contact coagulation is carried out or not, water after mechanical filters is checked for coagulant content.
Dear sirs, if you have a need to implement water purification using coagulants to bring water quality to certain standards, please make a request to the company’s specialists Waterman. We will develop for you the optimal technological scheme for water purification.
Lyophobic colloidal solutions, as thermodynamically unstable systems, can collapse spontaneously or under the influence of external influences. The destruction of colloidal solutions begins with their coagulation.
Coagulation is the process of aggregation of colloidal particles with the formation of larger aggregates due to the loss of aggregative stability by the colloidal solution.
As a result of coagulation, the enlarged particles of the dispersed phase easily sediment, and stratification of the system occurs. Thus, the cause of coagulation is the loss of aggregative stability of the colloidal solution, and the consequence of coagulation is a decrease in its sedimentation stability.
In practice, coagulation can be caused by various external influences: adding small amounts of electrolyte, concentrating a colloidal solution, changing temperature, exposure to ultrasound, electromagnetic field, etc.
The phenomenon of coagulation underlies many pathological processes occurring in living systems. Coagulation of colloidal solutions of calcium phosphate and cholesterol in the blood leads to the formation of sediments and their deposition on the inner surface of blood vessels (atherosclerotic changes in blood vessels).
Coagulation occurs during the process of blood clotting. Blood clotting plays two opposing roles in the body: on the one hand, it reduces blood loss when tissue is damaged, on the other, it causes the formation of blood clots in the circulatory system. Blood clotting is a very complex enzymatic process. At the same time, an anticoagulant system operates in the blood, the basis of which is heparin, a blood anticoagulant.
The nature of blood must be taken into account when preserving it. Since calcium cations promote blood clotting, they are removed from blood intended for preservation using various physicochemical methods. For example, the addition of sodium citrate precipitates calcium, after which the blood is kept refrigerated, remaining suitable for transfusion for 30 days. Whole blood can also be decalcified by ion exchange using Na-cation exchangers.
Coagulation under the influence of electrolytes. In biological systems, coagulation is of greatest practical importance when adding small amounts of electrolyte, since colloidal solutions of cells and biological fluids are in contact with electrolytes. Any electrolyte can cause coagulation of a colloidal solution. However, each electrolyte requires its own minimum concentration, called the coagulation threshold (C pc).
Coagulation threshold is the minimum amount of electrolyte that must be added to a colloidal solution to cause obvious coagulation (noticeable to the eye) - clouding of the solution or a change in its color. The coagulation threshold can be calculated using the formula:
where Sel is the initial concentration of the electrolyte solution; Vel is the volume of electrolyte solution added to the colloidal solution; Vкp is the volume of colloidal solution.
The reciprocal of the coagulation threshold is called coagulating effect(y): y=1/Spk
The coagulating effect of electrolytes on colloidal solutions with an ionic stabilizer is subject to Schulze-Hardy rule: coagulation of colloidal solutions is caused by any ions that have a charge sign opposite to the charge of the granules. The higher the charge of the coagulant ion, the stronger the coagulating effect of ions (y).
The coagulating effect of a coagulant ion is directly proportional to its charge to the sixth power: y = f(z 6). For example, coagulation of an AgI sol with negatively charged granules (potential-determining ions - anions I -) occurs due to the action of positively charged ions. Therefore, when solutions of NaCl, CaCl 2, AlCl 3 are added to this sol, the coagulating effect of Na +, Ca 2+, Al 3+ cations will increase sharply; y(Na +):y(Ca 2+):y(Al 3+) = 1:64:729. Coagulation of the AgI sol with positively charged granules (potential-determining ions-cations Ag +), on the contrary, occurs due to negatively charged ions. Adding solutions of KCl, K 2 SO 4, K 3 to the sol will cause an increase in the coagulating effect of anions in the following order: y(Cl -):y(SO 4 (2-)):y 3- = 1:64:729.
There are deviations from the Schulze-Hardy rule, since the coagulating effect of an ion, in addition to the charge, is influenced by the radius of the coagulating ion, as well as the nature of the ion accompanying the coagulant ion.
The strong influence of the electrolyte on the coagulation of colloidal solutions should be taken into account when introducing salt solutions into living organisms. In this case, not only the concentration, but also the charge of the introduced ions is important. Thus, a physiological solution of sodium chloride (0.9%) cannot be replaced with an isotonic solution of magnesium sulfate, since this salt contains doubly charged ions Mg 2+ and SO 4 (2-), which have a higher coagulating effect than Na + and Cl - ions .
When injecting an electrolyte into muscle tissue or human blood, it is necessary to inject it gradually, slowly, so as not to cause coagulation of biological colloid systems. Rapid administration of an electrolyte, due to the low rate of diffusion in the blood or muscle tissue, leads to the accumulation of the electrolyte, a local (local) excess of its threshold concentration and causes coagulation of biosubstrates, which is difficult to stop. With slow administration, the electrolyte has time to be carried away with the bloodstream and diffuse into neighboring tissues, so the threshold concentration is not reached and coagulation does not occur. This phenomenon in living tissues is called "addiction".
Coagulation mechanism. The role of electrolytes during coagulation is to reduce the disjoining pressure between approaching colloidal particles. This can happen in two ways: by reducing the surface charge of the solid phase (the surface charge of the core), i.e. due to a decrease in the interfacial potential Фмф, or due to a decrease in the thickness (compression) of the ionic atmospheres of micelles while the surface charge of their nuclei remains unchanged. In this regard, two types of coagulation are possible: neutralization and concentration.
Neutralization coagulation occurs under the influence of an electrolyte, which chemically interacts with potential-determining ions, binding them into a strong compound (for example, precipitating them) and thereby reducing the charge on the surface of the nucleus. Neutralization coagulation is observed, for example, when K 2 S is added to a colloidal solution of AgI with positively charged granules (potential-determining ions - Ag + cations). A reaction occurs between the coagulating anions S 2- and the potential-determining cations Ag + with the formation of the poorly soluble compound Ag 2 S, which leads to the destruction of the AgI micelle:

As a result of the binding of potential-determining cations Ag +, the interfacial potential F mf decreases and the number of NO 3 (-) counterions necessary to compensate for the charge on the surface of the nucleus decreases. Thus, the ionic atmospheres around the nuclei become thinner, the disjoining pressure between approaching particles decreases, and this in turn leads to their sticking together into larger aggregates.
Concentration coagulation occurs under the influence of an electrolyte, which does not chemically interact with stabilizer ions and does not change the surface charge of the micelle core. However, in this case, the coagulating effect is exerted by those ions of the added electrolyte, which are counterions for these micelles, since by increasing their concentration they penetrate into the granule, compressing (densifying) the ionic atmosphere of the micelle around the core. Concentration coagulation occurs at a constant interfacial potential Ф mf, but is usually accompanied by a decrease in the ζ-potential. Concentration coagulation is observed, for example, when nitrates are added to a colloidal solution of AgI, the micelles of which contain NO 3 (-) counterions:

As the concentration of added NO 3 (-) ions increases, they promote the introduction of counterions of the diffuse layer into the adsorption layer. In this case, the diffuse layer is compressed, and a state may occur in which the diffuse layer disappears completely and the granule becomes electrically neutral. In this state, the disjoining pressure between approaching particles is minimal, and this leads to the particles sticking together into larger aggregates.
Since the charge of the granules under these conditions is 0, in an electric field they do not acquire directional movement towards the electrodes, since the granule is in an isoelectric state.
The isoelectric state is the state of colloidal particles in which the electrokinetic potential ζ is equal to 0 and which is characterized by the absence of directional movement of granules in an electric field.
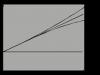
In the aggregation-stable state of the colloidal solution, the value fluctuates between 50-70 mV. When the ζ-potential decreases under the influence of an electrolyte to 25-30 mV, no external changes (turbidity or color changes) are observed in the system, since the coagulation rate is still very low, as a result of which this stage (I) of coagulation is called “latent” coagulation (Fig. .6.10). Further addition of electrolyte above Spk causes even greater compression of the diffuse layer and a decrease in the ζ-potential, which is accompanied by turbidity of the solution, and “explicit” coagulation begins. Initially, the coagulation rate increases rapidly (stage II), and then becomes constant when the ζ-potential value becomes zero and the rapid coagulation stage begins (III).
Coagulation with electrolyte mixtures. In practice, coagulation is often caused by the action of a mixture of electrolytes. In this case, there are three possible options for interaction between electrolytes: additive action, antagonism and synergism.
Rice. 6.10. Effect of electrolyte concentration on coagulation rate
Additivity- this is the summation of the coagulating effect of ions that cause coagulation.
The additive effect occurs in cases where electrolytes containing coagulating ions do not react chemically with each other. For example, a mixture of KCl and NaNO3 salts exhibits an additive effect in relation to colloidal solutions with both negatively and positively charged granules. In the first case, coagulation is caused by K + and Na + cations, in the second - by Cl - and NO 3 (-) anions.
Antagonism- this is a weakening of the coagulating effect of one electrolyte in the presence of another.
Pb 2+ + 2Cl - = PbCl 2 ↓
Antagonism of action is observed in cases where, as a result of a chemical reaction between electrolytes, coagulating ions are bound into an insoluble compound (precipitate) or into a stable complex that does not have coagulating ability. For example, the coagulating effect of Pb 2+ cations in relation to negatively charged granules is weakened in the presence of NaCl, since a reaction occurs as a result of which the concentration of coagulating Pb 2+ ions in the solution decreases due to the precipitation of PbCl 2:
Synergy- this is an increase in the coagulating effect of one electrolyte in the presence of another.
Synergistic action is possible when a chemical interaction occurs between electrolytes, resulting in the formation of a multiply charged ion with a very high coagulating ability. For example, the coagulating effect of FeCl 3 and KCNS in relation to positively charged granules (coagulating ions Cl (-) and CNS -) is enhanced many times, since a reaction occurs that results in the formation of multiply charged 3- anions that exhibit high coagulating ability:
FeCl 3 + 6KCNS → K 3 + 3KCl
When using electrolytes in laboratory and medical practice, the possibility of coagulation in biological media must always be taken into account. Thus, when introducing various medicinal substances into the body (in the form of injections), you should first make sure that these substances are not synergists in order to avoid possible coagulation. On the other hand, when purifying industrial waters, the antagonism of introduced electrolytes, which prevents the destruction of colloidal contaminants, can be harmful.
In natural waters, as well as in industrial wastewater, coagulation often occurs as a result of mixing dispersed systems containing dissimilar particles. Heterocoagulation called coagulation of colloidal solutions containing dissimilar particles that differ in chemical nature, sign or magnitude of charge.
A special case of heterocoagulation is mutual coagulation- adhesion of differently charged granules of colloidal solutions. In this case, coagulation occurs the more completely, the more completely the charges of the granules are neutralized.
Heterocoagulation is widely used in practice in connection with the problem of purifying natural and industrial waters. Aluminum or iron salts (3), which are good coagulants, are added to water containing colloidal impurities. As a result of hydrolysis, these salts give poorly soluble hydroxides Al(OH) 3 or Fe(OH) 3, forming colloidal solutions with positively charged granules. As a result, coagulation occurs, accompanied by the formation of flakes from aggregated heterogeneous micelles, which precipitate.
During the process of coagulation, associated with the loss of aggregative stability, the destruction of the colloidal solution occurs, accompanied by the precipitation of a precipitate - coagulate. However, if aggregative stability is restored to the coagulum, then the reverse process can occur - peptization.
Peptization called the reverse process of coagulation - the transformation of the sediment formed as a result of coagulation into a stable colloidal solution.
Peptization can be carried out in two ways, each of which leads to an increase in aggregative stability due to the restoration of fairly loose ionic atmospheres in micelles:
· washing the coagulate with a pure solvent (dispersion medium), which leads to the washing out of the system of ions that caused coagulation and loosening of the ionic atmospheres around the particles;
· by adding a special electrolyte-peptizer, the ions of which, adsorbed on the surface of coagulate particles, restore the loose ionic atmosphere around these particles and contribute to their transition to a colloidal state.
However, not every precipitate obtained during coagulation can be peptized. The most important conditions for effective peptization are as follows:
· only freshly obtained sediments are capable of peptization, since an increase in the duration of contact of particles of the dispersed phase with each other leads to a gradual compaction of the sediment and the displacement of the liquid phase from its structure;
· it is necessary to add small amounts of electrolyte-peptizer, otherwise coagulation may occur again;
· Peptization is promoted by stirring and heating.
The peptization process underlies the treatment of a number of pathological changes in the human body: resorption of atherosclerotic plaques on the walls of blood vessels, kidney and liver stones or blood clots in blood vessels under the influence of anticoagulants. In this case, it is necessary to take into account the timely introduction of medicinal substances (anticoagulants) into the blood: old blood clots in blood vessels, as well as hardened stones, are practically not peptized, i.e. do not dissolve.
6.9. Coarsely dispersed systems: suspensions, emulsions, aerosols
Coarsely dispersed systems are divided into three groups: emulsions, suspensions and aerosols.
Emulsions– these are dispersed systems with a liquid dispersion medium and a liquid dispersed phase.
They can also be divided into two groups:
1. direct – drops of a non-polar liquid in a polar medium (oil in water);
2. reverse (water in oil).
A change in the composition of emulsions or external influences can lead to the transformation of a direct emulsion into a reverse emulsion and vice versa. Examples of the most famous natural emulsions are milk (forward emulsion) and oil (inverse emulsion). A typical biological emulsion is fat droplets in the lymph. In chemical technology, emulsion polymerization is widely used as the main method for producing rubbers, polystyrene, polyvinyl acetate, etc.
Suspensions– these are coarse systems with a solid dispersed phase and a liquid dispersion medium.
A special group consists of coarsely dispersed systems, in which the concentration of the dispersed phase is relatively high compared to its low concentration in suspensions. Such dispersed systems are called pastes. For example, dental, cosmetic, hygiene, etc., that are well known to you from everyday life.
Aerosols– these are coarsely dispersed systems in which the dispersion medium is air, and the dispersed phase can be liquid droplets (clouds, rainbows, hairspray or deodorant released from a can) or particles of a solid substance (dust cloud, tornado).
Colloidal systems occupy an intermediate position between coarse systems and true solutions. They are widespread in nature. Soil, clay, natural waters, many minerals, including some precious stones, are all colloidal systems.
Colloidal systems are of great importance for biology and medicine. The composition of any living organism includes solid, liquid and gaseous substances that are in a complex relationship with the environment. From a chemical point of view, the body as a whole is a complex collection of many colloidal systems.
Colloidal systems are divided into sols (colloidal solutions) and gels (jelly).
Most biological fluids of a cell (cytoplasm, nuclear juice - karyoplasm, contents of vacuoles) and the living organism as a whole are colloidal solutions (sols).
For sols The phenomenon of coagulation is characteristic, i.e. adhesion of colloidal particles and their precipitation. In this case, the colloidal solution turns into a suspension or gel. Some organic colloids coagulate when heated (egg whites, adhesives) or when the acid-base environment changes (digestive juices).
Gels are colloidal systems in which particles of the dispersed phase form a spatial structure.
Gels are dispersed systems that you encounter in everyday life.
Over time, the structure of the gels is disrupted and liquid is released from them. Happening syneresis– spontaneous decrease in the volume of the gel, accompanied by the separation of liquid. Syneresis determines the shelf life of food, medical and cosmetic gels. Biological syneresis is very important when making cheese and cottage cheese.
In appearance, true and colloidal solutions are difficult to distinguish from each other. To do this, use Tyndall effect– formation of a cone of a “luminous path” when a beam of light is passed through a colloidal solution. Particles of the dispersed phase of the sol reflect light with their surface, but particles of the true solution do not. You can observe a similar effect, but only for an aerosol rather than a liquid colloid, in a cinema when a beam of light from a movie camera passes through the dusty air of the auditorium.
6.10. Electrokinetic phenomena in dispersed systems: electrophoresis, electroosmosis
An electrical charge can occur on any solid surface that is in contact with a liquid. The value of the specific charge is relatively small: for example, for clay at the boundary with water it is several tens of milliculombs, so the surface of a piece of clay weighing 1 kg, equal to hundredths of a square meter, will have a negligible electrical charge. Clay particles with a total mass of 1 kg create a surface area millions of times larger than a solid piece, which leads to a sharp increase in surface charge. The appearance of a significant surface charge is the cause of special electrokinetic phenomena characteristic only of dispersed systems.
Electrokinetic phenomena are those that occur when an electric field acts on dispersed systems and as a result of the movement of particles of the dispersed phase or dispersion medium. Despite the differences in electrokinetic phenomena, they are all associated with the presence of a double electric layer and are determined by the ζ-potential, which is why it is called electrokinetic.
An external electric field causes such electrokinetic phenomena of dispersed systems as electrophoresis and electroosmosis.
Electrophoresis is the movement of particles of the dispersed phase relative to the dispersion medium under the influence of an electric field. The electrophoresis scheme is shown in Fig. 6.11, where the dispersed phase particle is shown on an enlarged scale for clarity. When an external electric field is applied, particles of the dispersed phase begin to move towards the electrode, the sign of the charge is opposite to the sign of the ζ-potential; The direction of particle motion in the figure is shown by an arrow.
The movement of particles during electrophoresis is due to the attraction of unlike charges. The diffuse layer does not prevent the interaction of unlike charges. Counterions in this layer are mobile, distributed unevenly and are not able to screen the effect of an external electric field on particles of the dispersed phase. The movement of particles occurs along the sliding boundary.
During electrophoresis, the spherical symmetry of the diffuse layer of counterions is broken, and it begins to move in the direction opposite to the movement of the particles. The oppositely directed flow of particles in the diffuse layer slows down the movement of particles. This effect is called electrophoretic inhibition (short arrow in Fig. 6.11).

Rice. 6.11. Electrophoresis scheme:
During electrophoresis, particles of the dispersed phase move in the direction of the electric field lines. Electrophoresis is used to obtain new materials, apply coatings, purify substances from impurities, and isolate products. In medicine, electrophoresis is used to administer drugs. A tampon moistened with a solution of the drug is placed on the patient’s skin, and electrodes are placed on top, to which a low potential that is safe for the body is applied. During this procedure, particles of the drug are transferred into the tissues of the human body under the influence of an electric field.
Electroosmosis is called the movement of a dispersion medium under the influence of an external electric field (Fig. 6.12). The movement of the dispersion medium is caused by the attraction of unlike charges. It often occurs in capillaries and in the channels of porous bodies. When the ζ potential is negative, the positively charged counterions in the diffuse layer are attracted to the negative electrode. The counterions carry with them the liquid that makes up the dispersion medium. As a result of this, liquid movement occurs, and the movement of the liquid dispersion medium relative to the particles of the dispersed phase, as in the case of electrophoresis, occurs along the sliding boundary.

Rice. 6.12. Electroosmosis circuit
1 - dispersed system; 2 - partition
Electroosmosis is used, for example, for dehydration of wood and other porous materials: construction materials, soil, food, raw materials for the food industry, etc. The wet mass is placed between the electrodes, and water, depending on the structure of the DEL, moves to one of them and is collected in a special container .
To carry out electrophoresis or electroosmosis, an external electric field is required, i.e. the movement of particles during electrophoresis or the medium during electroosmosis is a consequence of the influence of this field.
It should be noted that the phenomenon of electrophoresis is characteristic mainly of colloidal solutions (sols), i.e. for systems in which the particle size of the dispersed phase does not exceed 0.1 microns. Electroosmosis can be observed not only in relation to colloidal solutions, which are highly dispersed systems, but also in relation to medium and coarsely dispersed systems.
Coagulation of colloidal solutions.
Coagulation
Colloidal systems have different stability. All of them strive to reduce the free surface energy by reducing the specific surface of colloidal particles, which occurs when they tend to unite.
The specific surface area of these particles is very large, which is why they have a large excess of surface energy, which, in turn, leads to thermodynamic instability of colloidal systems.
The process of combining colloidal particles into larger aggregates is called coagulation.
Disjoining pressure
According to the theory of coagulation B.V. Deryagin and L.D. Landau, during Brownian motion, colloidal particles freely approach each other to a distance of about 10 -5 cm, but their further approach is prevented by the so-called disjoining pressure that occurs in thin layers of water located between two surfaces.
Disjoining pressure is the excess (compared to hydrostatic) pressure acting from a thin layer of liquid on the bounding surfaces.
In sols, it is caused mainly by the mutual repulsion of counterions of the diffuse layer of approaching particles and, in addition, by the forces of molecular interaction between the surfaces of these particles and water molecules.
Changes in the properties of water around colloidal particles
Under the influence of electrostatic fields created by ions located on the surface of colloidal particles, the water molecules adjacent to them become more polarized and arranged in a more orderly manner, which, in particular, strengthens the connection not only between these water molecules, but also between them and the colloidal particles.
As a result, the layer of water adjacent to the particle acquires special properties ( increased viscosity and elasticity), which prevents particles from combining.
Overcoming disjoining pressure
If the particles have sufficient energy to overcome the wedging pressure, then at a distance equal to the diameter of the particles, i.e. approximately 10 -7 - 10 -8 cm, the forces of intermolecular attraction begin to predominate, and the particles come together.
Only a very small number of collisions result in particle aggregation, which is why many sols are stable.
If you lower the charge of colloidal particles, then such particles will coagulate more easily and more strongly.
Colloidal particles whose granule charge is zero coagulate at the highest speed, i.e. particles in an isoelectric state.
The lack of charge on the granule means that the particle has no counterions in the diffusion layer and, therefore, their aqueous shell.
It also turned out that polydisperse sols coagulate faster than monodisperse sols and that the shape of the particles matters for this process: rod-shaped particles coagulate at the highest speed.
When two sol particles (the so-called first order particles) a larger one is formed second order particle, which can combine with another first-order particle, forming third order particle, which again attaches a first-order particle and turns into fourth order particle etc.
Calculations have shown that the joining of particles of first orders occurs more easily than the joining of particles of higher orders.
The sum of all particles in the ash during coagulation continuously decreases, and if the number of initial particles is of the first order n1 decreases all the time, then the number of second-order particles n2 first increases and then decreases. Slightly behind in time n2 the number of third-order particles increases n3, which, having passed its maximum, begins to fall. At this time, the number of particles of the next order increases, etc.
Particle sedimentation
As a result, when coagulation loose aggregates of various sizes are formed, in which the particles are loosely connected to each other.
Large aggregates begin to sink to the bottom of the vessel under the influence of gravity. A process of sedimentation occurs.
Sedimentation(sedimentation) - settling of dispersed phase particles in a liquid or gas under the influence of a gravitational field or centrifugal forces.
The rate of sedimentation depends on the size and density of particles, their charge, solution viscosity, etc.
Particles in an isoelectric state settle faster, since the charge does not interfere with their coagulation and sedimentation.
Using Centrifuges for Sedimentation
To speed up the sedimentation process, they are widely used centrifuges. The centrifugal force generated by them causes the particles to settle faster. With a sufficient number of revolutions, even non-coagulated particles can be deposited.
At constant temperature, solvent viscosity, particle charge, etc. the rate of their deposition depends on differences in their mass and size, thanks to which the molecular weight of these particles can be calculated.
With help ultracentrifuge, developing speeds of tens of thousands of revolutions per minute, the molecular weights of many proteins and other organic compounds were determined.
Change in coagulation rate
Spontaneous coagulation of many sols often occurs slowly. It can be accelerated by increasing the speed of particle movement.
This will help them overcome disjoining pressure. The acceleration of particle motion can be caused, for example,. increasing the temperature of the solution also leads to an acceleration of its coagulation, since with increasing concentration the number of effective collisions between micelles increases.
The coagulation process is very sensitive to the addition electrolytes.
Electrolyte- a substance that conducts electric current due to dissociation into ions.
Examples of electrolytes include aqueous solutions of acids, salts and bases, etc.
Small amounts of electrolytes can dramatically accelerate the rate of coagulation. Consequently, on the one hand, electrolytes are necessary to stabilize sols, and on the other hand, their excessive addition leads to coagulation of the sols.
The influence of different electrolytes on this process is different.
Dependence of coagulation on the charge value of the electrolyte ion The coagulating effect of electrolytes depends on the magnitude of the charge of the ion, which is opposite to the charge of the colloidal particle. Coagulates at the highest speed
electrically neutral particles ζ . This state of a particle, charged before the start of coagulation, for example, positively, will become possible if all counterions of the diffuse layer, charged negatively, are moved to the adsorption layer.
The higher the concentration of the added electrolyte, the more the diffusion layer will be compressed, the smaller it will become-potential and coagulation will go faster.
A- before coagulation begins, the granule is positively charged;
B - the granule has become electrically neutral, coagulation proceeds at maximum speed. With a sufficient concentration of the electrolyte, almost all of its counterions will end up in the adsorption layer, and the charge of the particle will drop to zero. The absence of a diffuse layer will cause a significant decrease in wedging pressure and
coagulation
will go at maximum speed.
The coagulating effect of ions increases sharply with an increase in the number of their charges in a progression that is roughly taken as the ratio of sixth powers of the number of ion charges: 1: 2 6: 3 6, etc.
In reality, due to the influence of a number of factors, this ratio turns out to be smaller. |
|||
From the data in the tables below it follows that the coagulating ability of doubly charged ions is tens of times, and that of triply charged ions is hundreds of times higher than that of singly charged ions. | As 2 S 3 | Electrolyte
| coagulation
|
|---|---|---|---|
compared with Na+ |
|||
From the data in the tables below it follows that the coagulating ability of doubly charged ions is tens of times, and that of triply charged ions is hundreds of times higher than that of singly charged ions. | As 2 S 3 | Electrolyte
| coagulation
|
|---|---|---|---|
The coagulating abilities of the same ions in relation to another sol will have different values. But the difference in these values will not be very large. Therefore, it is possible to arrange equally charged ions in lyotropic series
, showing the order in which their coagulating ability decreases for all oppositely charged sols.
Positive ions:
Cs + > Rb + > K + > Na + > Li +
Ba 2+ > Sr 2+ > Ca 2+ > Mg 2+
Negative ions:
Cl - > Br - > NO 3 - > I - > CNS -
Mechanism of coagulating action of electrolytes
Three factors can be distinguished in the mechanism of the coagulating action of electrolytes.
1. Compression of the diffusion layer.
The greater the charge of the coagulating ions, the more they compress the diffuse layer of counterions.
2. Adsorption of ions on a colloidal particle.
Selective adsorption of those ions of the added electrolyte that have a charge opposite to the granule occurs on the colloidal particle. The higher the charge of the ions, the more intensely they are adsorbed. The accumulation of ions charged opposite to the particle that occurs in the adsorbed layer is accompanied by a corresponding decrease in ζ-potential and therefore diffuse layer ..
And this, in turn,
increases the rate of coagulation 3. The process of ion exchange adsorption..
In addition to the compression of the diffuse layer and the adsorption of ions, during the coagulation of sols with electrolytes, the process of ion exchange adsorption occurs, in which counterions of the adsorption layer are exchanged for similarly charged ions of the added electrolyte If the charge of the latter is higher.
than that of counterions, then such a replacement leads to a significant
decrease in ζ-potential
Thus, all three processes that reduce the charge of the granule and affect coagulation are more effective, the higher the charge of the coagulating ion. This partially explains the difference in the coagulating effect of ions with different charge values. The main reason for particle coagulation is
decrease in disjoining pressure
to such a level that it ceases to prevent the unification of particles. Change of polarity of colloidal particles.
With a significant increase in the concentration of added multiply charged ions, they can be adsorbed on colloidal particles in such large quantities that the granules can not only become electrically neutral, but also, in general, change your polarity In this case, the granules acquire the sign of the charge of the excess adsorbed ions of the added electrolyte and again become
sustainable FeCl3 a decrease in the negative charge of colloidal platinum particles and their coagulation are observed.
An increase in the amount of this electrolyte leads to a change in the polarity of the platinum particles, which acquire a positive charge.
Even larger quantities FeCl3 will again have a coagulating effect.
This alternation of states of electrical neutrality and charge of particles is called alternation of coagulation zones or phenomenon of irregular rows. It is not observed with all sols and not with all electrolytes.
Kinetics of coagulation. Latent and obvious coagulation.
If an electrolyte is slowly added to a colloidal solution, then its first portions do not affect the sol.
As the electrolyte concentration increases, the formation of particles of lower orders (II, III, etc.) begins, which occurs unnoticed by the naked eye and is therefore called hidden coagulation.
A further increase in the electrolyte concentration leads to the progressive development of the coagulation process, an increase in its speed and is accompanied by the appearance of particles of higher orders.
The sol undergoes visible changes: it becomes cloudy or its color changes. In this case, the value ζ -particle potential decreases.
This stage of the process is called obvious coagulation. The transition of latent coagulation to overt coagulation is called coagulation threshold: it corresponds to the threshold electrolyte concentration, i.e. the minimum electrolyte concentration that causes obvious coagulation.
(This value is measured in millimoles per liter of sol.) ζ At that time - the potential still remains, but it usually does not exceed 30 mV and is called.
critical ζ-potential ζ However, the change in value ζ -potential does not always correspond to the process of particle coagulation.
Coagulation often begins at high values ζ -potential, and sometimes with a decrease in this potential, some sols even increase their stability.
This confirms that-potential is an important, but not the only determining factor in the stability of colloidal particles.
Apparent coagulation, in turn, is divided into two periods:
1. Period of slow coagulation. During this period, any increase in electrolyte concentration accelerates coagulation.
2. Period of rapid coagulation.
The lowest concentration of electrolyte that causes rapid coagulation is called coagulation concentration or rapid coagulation threshold.
During coagulation, along with a decrease in the number of particles and their enlargement, a number of properties of solutions change: the rate of diffusion and filtration of particles decreases, the rate of sedimentation increases, the intensity of scattered light changes, and at the same time the color of solutions, etc.
Coagulation with electrolyte mixtures
There are three possible cases of combined action of a mixture of two or more electrolytes on a colloidal solution:
1. Additivity - summation of the coagulating effect of electrolytes,
2. Antagonism - one electrolyte weakens the effect of another,
3. Synergism - one electrolyte enhances the effect of another.
If we consider each electrolyte separately from the others, then there is a concentration that will cause rapid coagulation of the sol. Let us take this amount of electrolyte, which causes rapid coagulation, to be 100% and now consider the joint work of two different electrolytes.
Additivity
In the case of additivity, an attempt to achieve coagulation of the sol with one of the electrolytes at a concentration of less than 100% will require the addition of an appropriate amount of the second.
For example, if one takes 70% of the coagulation concentration, then the second will need to add 30% ( in total 100%).
Antagonism
With antagonism, in the action of electrolytes, it turns out that 70% of the concentration of one of them no longer requires 30% of the coagulation concentration of the other, but more, for example, 55%.
Thus, the sum of their concentrations will become more than 100%.
Synergy
With synergism, in order to obtain rapid coagulation of the sol at 70% of the coagulation concentration of one electrolyte, it is enough to add, for example, 15% of the coagulation concentration of the second electrolyte.
The resulting sum of concentrations will be less than 100%.
When coagulating sols with mixtures of electrolytes, synergism or antagonism is usually observed. Aditivity is a rare phenomenon.
Phenomenon synergy may be due to the formation of multicharged complex ions from the added electrolytes, which have a strong coagulating effect.
Antagonism is explained, in particular, by the formation of these electrolytes or complex compounds - peptizers, or weakly dissociated particles that do not affect the colloidal solution.
Peptization- splitting of aggregates formed during coagulation of dispersed systems into primary particles under the influence of a liquid medium (for example, water) or special substances - peptizers.
In the case of the formation of peptizers, unreacted electrolyte ions act in the direction of coagulation of the sol, and the peptizer formed from the reacted ions again transfers the coagulated particles into the sol.
Sometimes peptizers are formed as a result of the interaction of colloidal particles with the added electrolyte.
Thus, when HCl is added to a Fe(OH)2 sol, it coagulates, but when HCl is slowly added, there is no coagulation.
As it was found out, with the slow addition of hydrochloric acid, the peptizer has time to form:
Fe(OH)2 + HCl → FeOCl + 2H2O
FeOCl → FeO + + Cl - This phenomenon is called.
addictive sol
Mutual coagulation If you add a sol with positively charged particles to a sol with negatively charged particles, then their.
mutual coagulation
At many water treatment plants, positively charged sols of aluminum or iron hydroxide are added to water containing negatively charged organic mixtures.
After mutual coagulation, the resulting flakes can be easily filtered using sand filters.
Biological significance of coagulation coagulation Processes And peptization
are of great importance for the life of organisms, since colloids of cells and biological fluids are also subject to coagulation and are constantly exposed to electrolytes.
To maintain the constancy of physical and chemical conditions in the body, it is necessary to maintain the constancy of not only the concentration of electrolytes, but also their qualitative composition. Indeed, if you prepare an isotonic solution not from NaCl , and from an equal concentration of multiply charged ions, for example, MgSO4 Indeed, if you prepare an isotonic solution not from.
, then doubly charged ions will have a significantly stronger coagulating effect on colloids than Indeed, if you prepare an isotonic solution not from Processes The phenomena of antagonism and synergism of electrolytes are also reflected in biological objects. It is known that the growth of wheat roots is suppressed by 0.12 M solutions
Coagulation CaCl2
, but at a certain ratio of these solutions, the negative effect of the mixture of electrolytes is eliminated.- the process of colloidal particles sticking together with the formation of larger aggregates due to the loss of aggregative stability by the colloidal solution.
Coagulation threshold
- the minimum amount of electrolyte that must be added to the colloidal solution to cause obvious coagulation (noticeable to the eye); - clouding of the solution or a change in its color. spk = sel·Vel / Vcr+Vel Where With el Where- initial concentration of the electrolyte solution; el V- volume of electrolyte solution added to the colloidal solution;
cr ability - the reciprocal value of the coagulation threshold, obeys the Schulze-Hardy rule.
Colloidal protection, its role in life. Peptization, biological role
Colloidal protection- increasing the aggregative stability of lyophobic sols when adding IUDs to them.
The mechanism is that around the micelles of the colloidal solution, adsorption shells are formed from flexible BMC macromolecules, which are amphiphilic and their hydrophobic sections face the particles of the dispersed phase, and the hydrophilic fragments face the water.
In this case, the system is lyophilized, the micelles acquire an additional factor of aggregative stability due to their own hydration shells from the BMC macromolecules.
- · good solubility of BMC in the dispersed medium of a colloidal solution and adsorbability of molecules on colloidal particles;
- · quite high concentration.
Thus, blood proteins prevent the precipitation and release of poorly soluble cholesterol and calcium salts on the walls of blood vessels, and also prevent the formation of stones in the urinary and bile ducts.
Peptization- process, reverse coagulation, i.e. transformation of the precipitate formed as a result of coagulation into a stable colloidal solution.
This is done in two ways:
- 1. washing the coagulate with a pure solvent (DS);
- 2. adding a special electrolyte-peptizer.
Conditions for effective peptization:
- · only freshly obtained sediments are capable of peptization;
- · it is necessary to add small amounts of electrolyte-peptizer;
- · Peptization is promoted by stirring and heating.
This process underlies the resorption of atherosclerotic plaques on the walls of blood vessels, kidney and liver stones or blood vessel thrombi.